BioTechniques News
Beatrice Bowlby

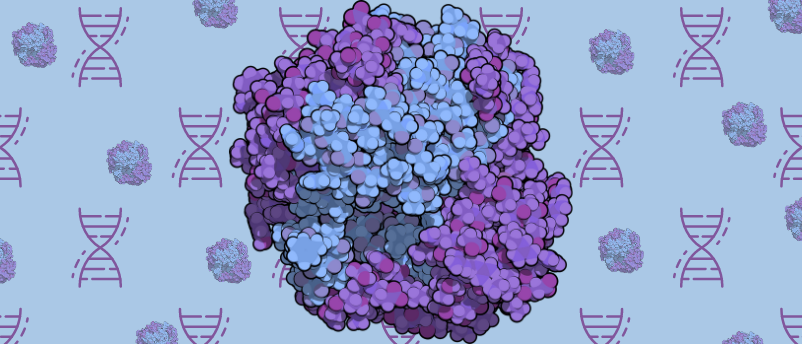
For the first time, the structure of a DNA enzyme complex implicated in infertility and cancer has been obtained using cryo-electron microscopy (cryo-EM).
A collaboration of researchers, led by Michael Trakselis (Baylor University, TX, USA), has used cryo-EM to capture the structure of the DNA enzyme complex called MCM8/9, which is part of the minichromosome maintenance protein (MCM) family. MCM8/9 is linked to infertility and several cancers including ovarian cancer, testicular cancer and colon cancer. Now that the structure has been uncovered, the researchers hope this will help them understand how this enzyme complex is connected to these diseases.
MCM proteins are DNA helicases that are essential for DNA replication and are involved in controlling replication times within a cell cycle. These proteins have been found to be overexpressed in cancerous tissues and cell lines; however, the underlying mechanism of their role in causing cancer is yet to be determined. Additionally, mutations of MCM8 and 9 have been linked to early menopause, amenorrhea (the absence of menstruation) and other signs of ovarian insufficiency, leading to infertility.
To find out how mutations in MCM8/9 lead to infertility or cancer, the structural information of this protein complex needed to be obtained.
 Cryo-EM reveals new structural elements in a key ciliary protein complex
Cryo-EM reveals new structural elements in a key ciliary protein complex
Scientists capture the protein complex responsible for transporting signaling molecules to cilia in unprecedented detail.
In order to use single-particle cryo-EM – “a massive instrument, but it works to image really tiny things,” commented Trakselis – to solve the structure, the researchers needed to find a way of making a pure and stable protein solution to attain precise structural information on the MCM8/9 complex. Through trial and error, the team co-expressed and purified the human protein from insect cells. This yielded a protein that was suitably pure and stable on which to perform cryo-EM imaging. Their efforts also revealed that MCM8/9 is an ATP-dependent DNA helicase.
“We described the basic structure–function relationship, but we don’t know what it does at the cellular level and in what context,” commented Trakselis.
This structural information is just the beginning. The researchers now have a roadmap to better understand the enzyme function and how mutations are linked to various diseases.
The post Structure of an enzyme complex linked to infertility has been uncovered appeared first on BioTechniques.
Powered by WPeMatico
