
ADJUVANT ADJUVANTMM#3001
For monoclonal antibody production
For highest fusion-yields in Mice. Particularly mild and simple to handle, just mix with antigen and inject. Available in tubes of 0.1ml for processing even the smallest amounts of Antigen without loss, as well as vials of 1ml and 5ml. All components are non-toxic and fully biocompatible.
Suggestion for Use
Storage: 4-8°C. Ready-made mixture should be stored frozen. Bring to room temperature prior to use.
Withdrawal: Use a sterile plastic syringe, mix with antigen solution and shake.
Dilution Rate: Standard rate is 1:1 with antigen solution, but other ratios are no problem as long as the recommended volume of adjuvant is used and the maximum injection volume at each site not exceeded.
Diluent: Use water as the diluent. If buffer is necessary, use HEPES, MOPS or glycine. Avoid polyvalent ions such as phosphate or citrate which can provoke coagulation of the positively charged particles. A pH between 5 and 6 has often proved to be more favourable than strictly neutral.
Route of application: Subcutaneous. Intraperitoneal and intramuscular application not recommended.
No.of injection sites: For optimum effect, more sites are always better.
Maximum injection volume per site: Recommendations are given by various Animal Protection Councils all over the world. [c/f. Lenaars, M. et al (1999) ATLA 27, 79-102. Also: Nicklas, W., Cußler, K., &Hartinger, J., T (1998) TVT, Tierschutzaspekte bei der Immunisierung von Versuchstieren].
Stability of Antigen- Stability of Antigen Adjuvant mix: No restriction, except antigen solution contains polyanionic compounds which could lead to precipitate.
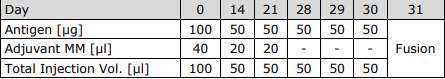
Schedule of Immunization
GERBU Adjuvants Compatibility
In the host organism the colloidal particles are rapidly rinsed away to the lymphatic system where they perform their action and cannot cause granulomas. All components are proven to be orally and parenterally nontoxic. GMDP has been found to be pyrogenic in doses over 10 µg/kg body mass.
Active Ingredients
The effectivity of Gerbu Adjuvant is based on the synergistic action of the muramyl glycopeptide and solid ultrafiltrable particles of slowly biodegradable lipids added in order to impart the positive electric charges beneficial for optimal efficiency. Equally important are the immunopotentiating, biocompatible emulsifiers and the carefully adjusted synergistic and stabilizing medium which surrounds the nanoparticles.
